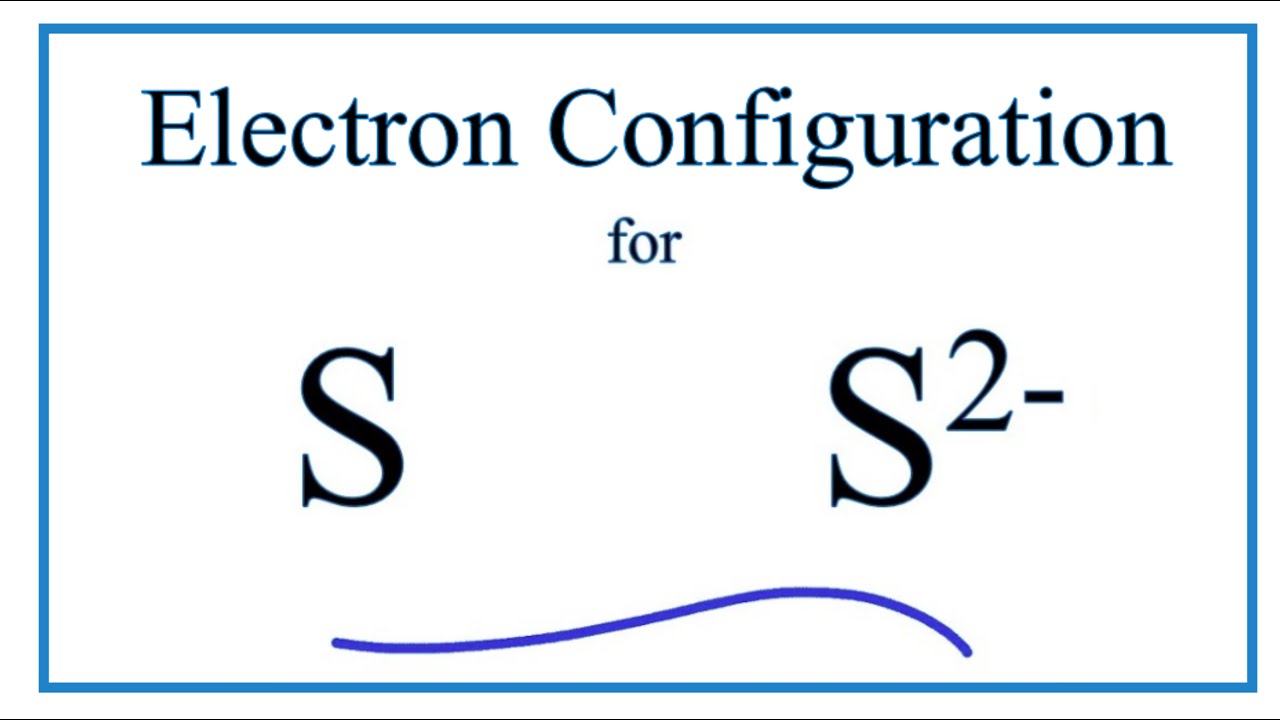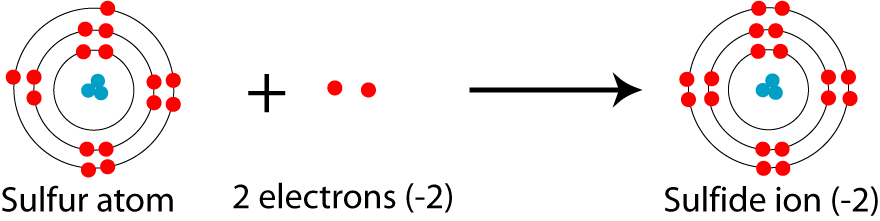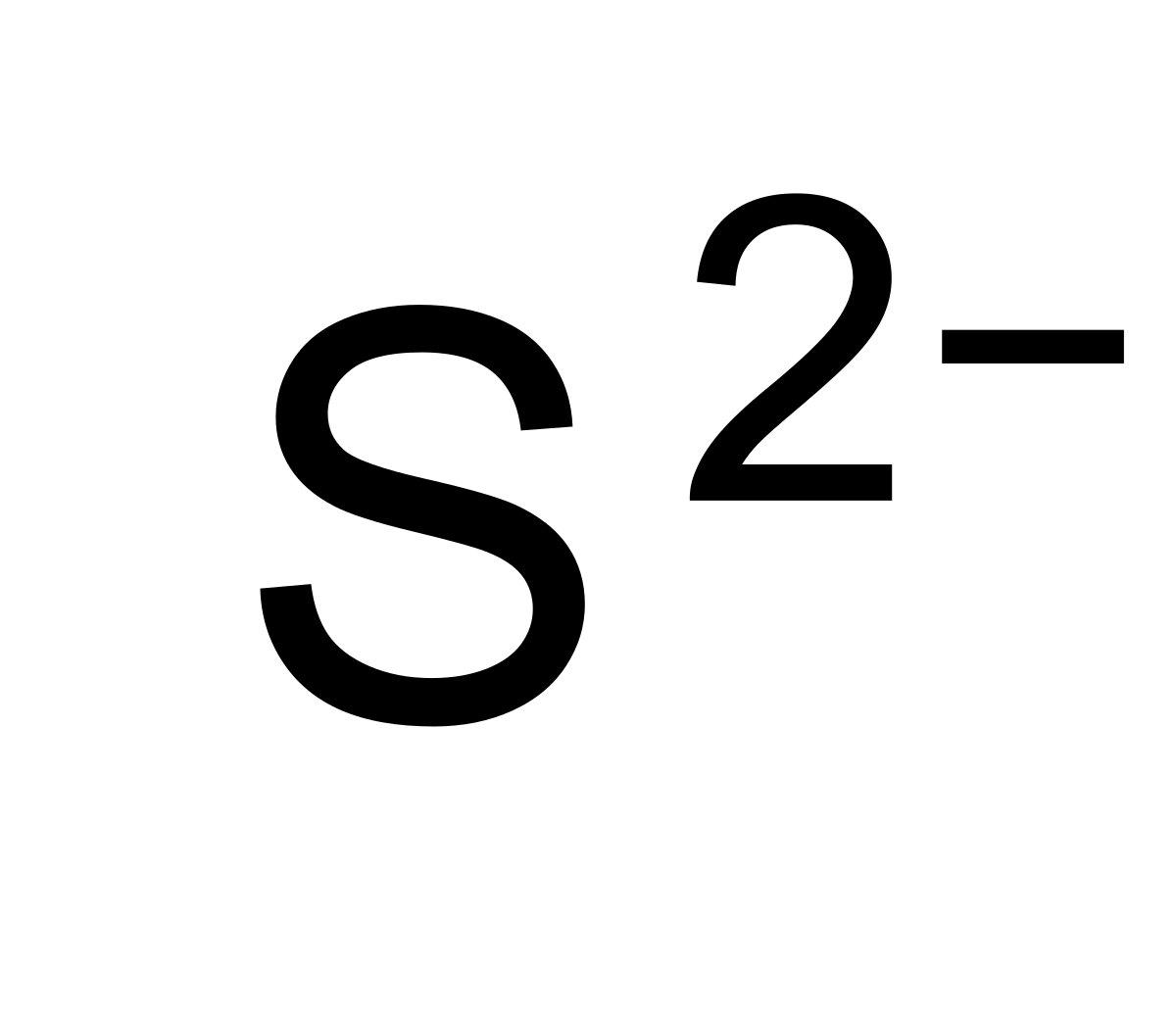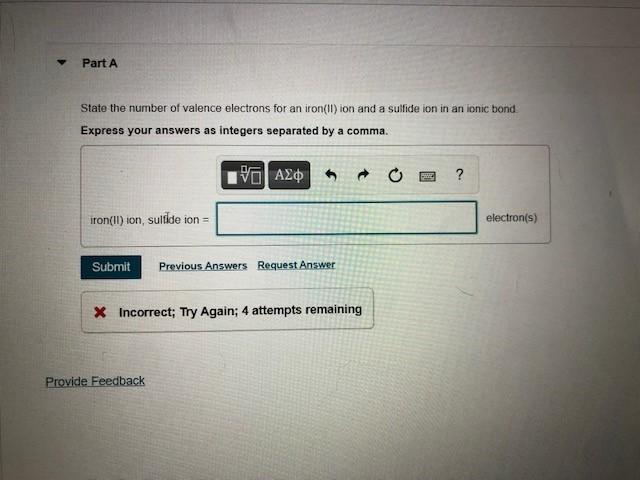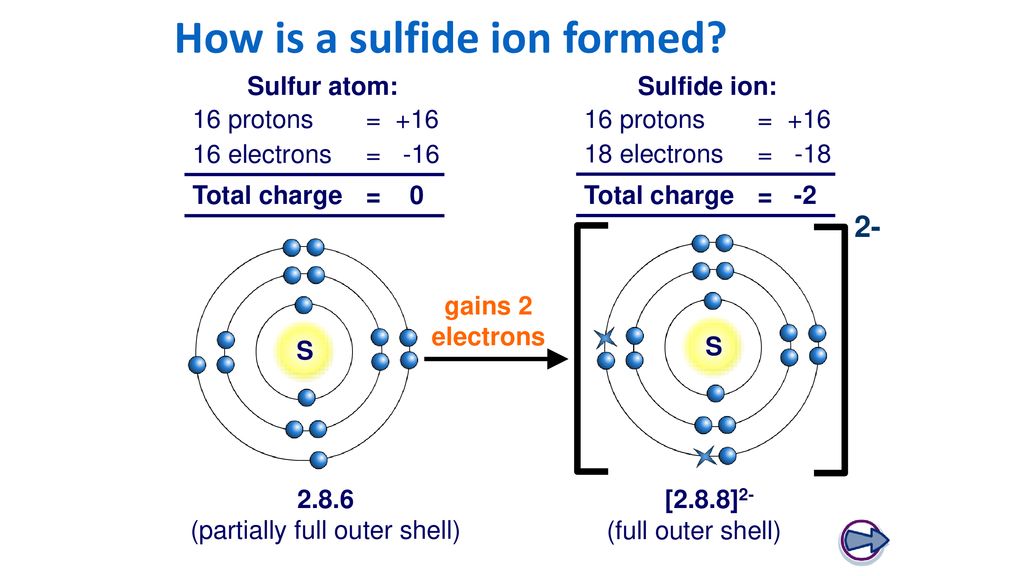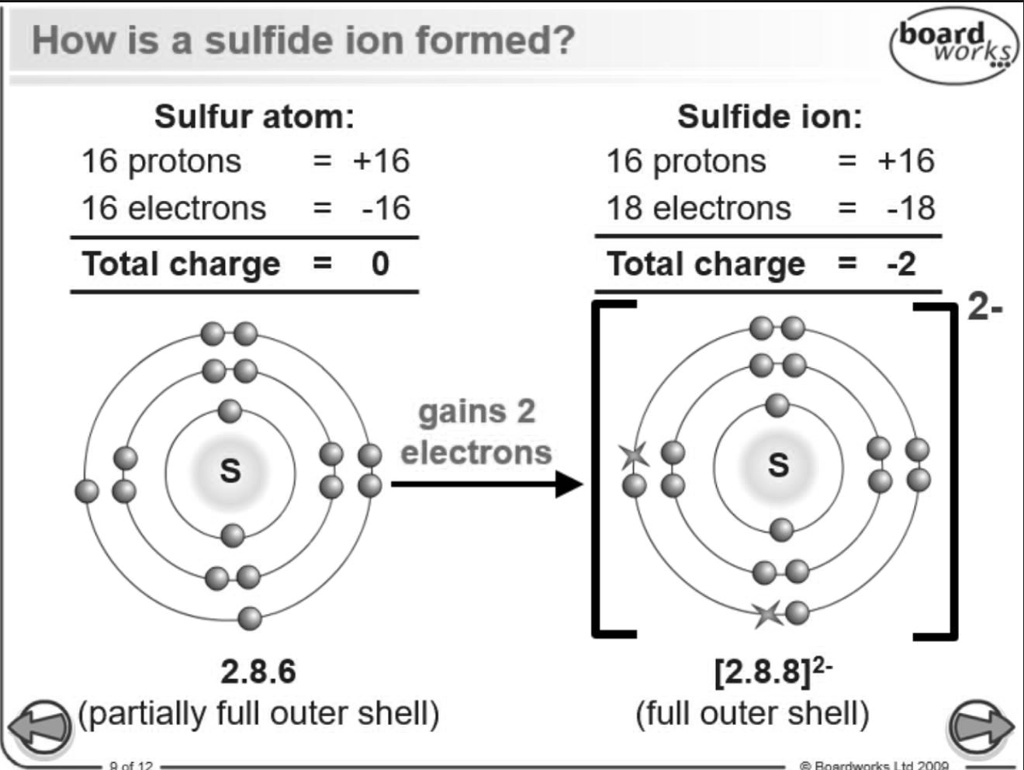
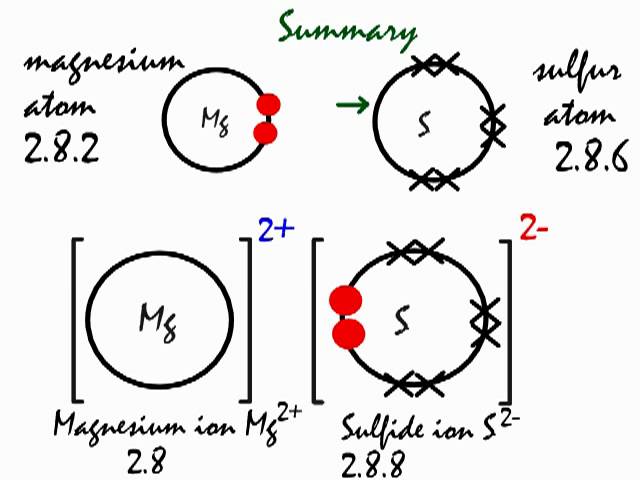
SOLVED: 'I thought in the third shell there are 18 electrons, but why are they gaining only 2 electrons?? Pls tell me... I have exam tomorrow.... How is a sulfide ion formed?

See answer: Which particle represents the size of the sulfide ion compared to the sulfur - Brainly.com
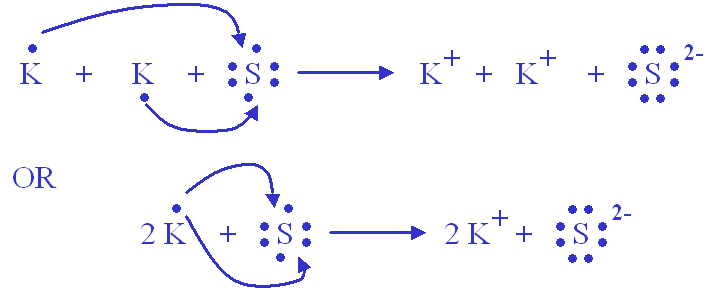
A potassium ion has a charge of 1+. A sulfide ion has a charge of 2- .Wha tis the chemical formula for potassium sulfide? | Socratic
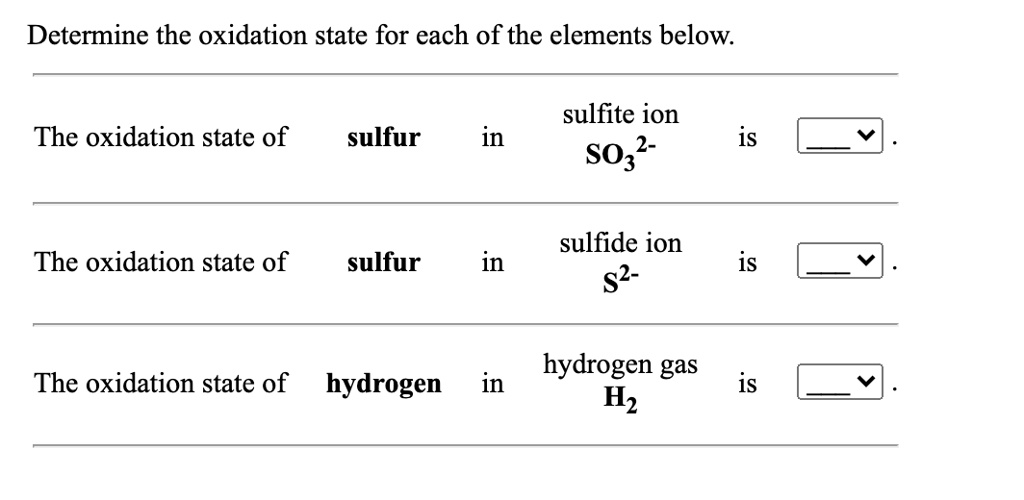
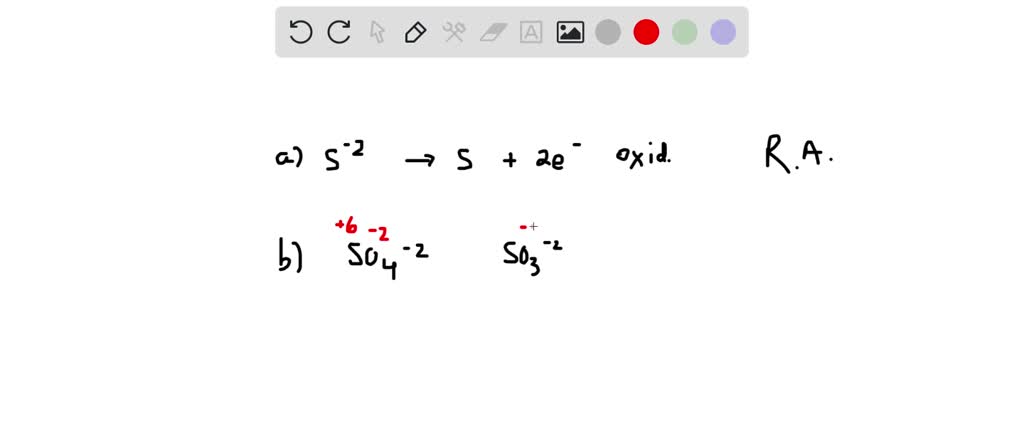
SOLVED: Determine the oxidation state for each of the elements below: sulfite ion 2- SO3 The oxidation state of sulfur in is sulfide ion s2- The oxidation state of sulfur in is

![Sulfid • einfach erklärt: Nachweis, Vorkommen · [mit Video] Sulfid • einfach erklärt: Nachweis, Vorkommen · [mit Video]](https://d1g9li960vagp7.cloudfront.net/wp-content/uploads/2021/07/Wordpress_Sulfid-Nachweis-mit-Bleiacetatpapier-1-1024x576.jpg)